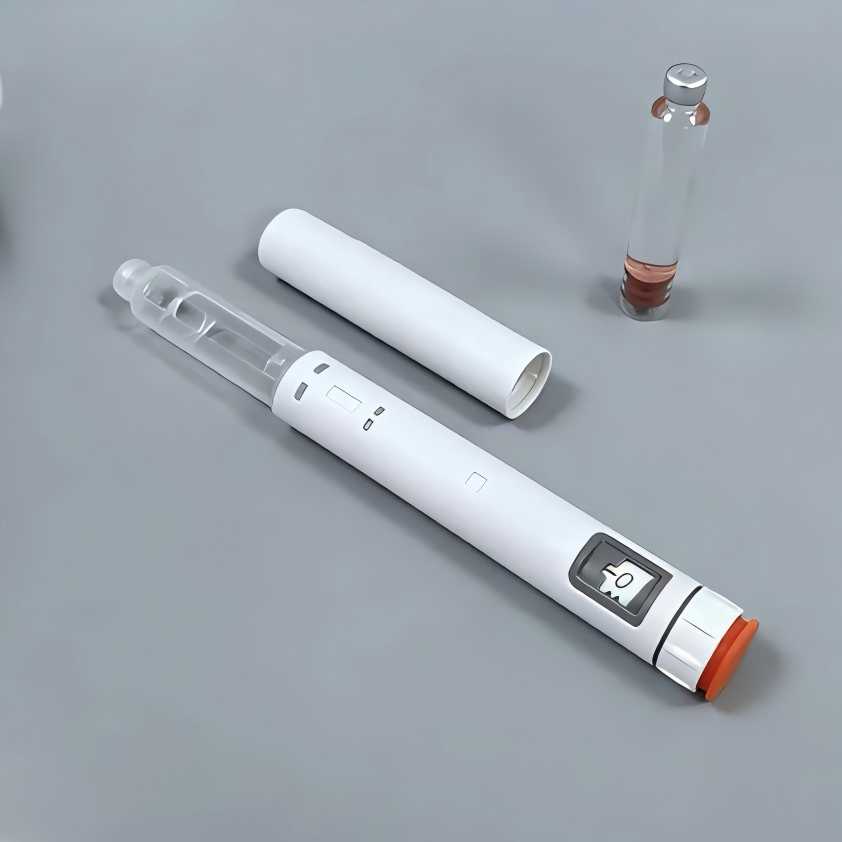
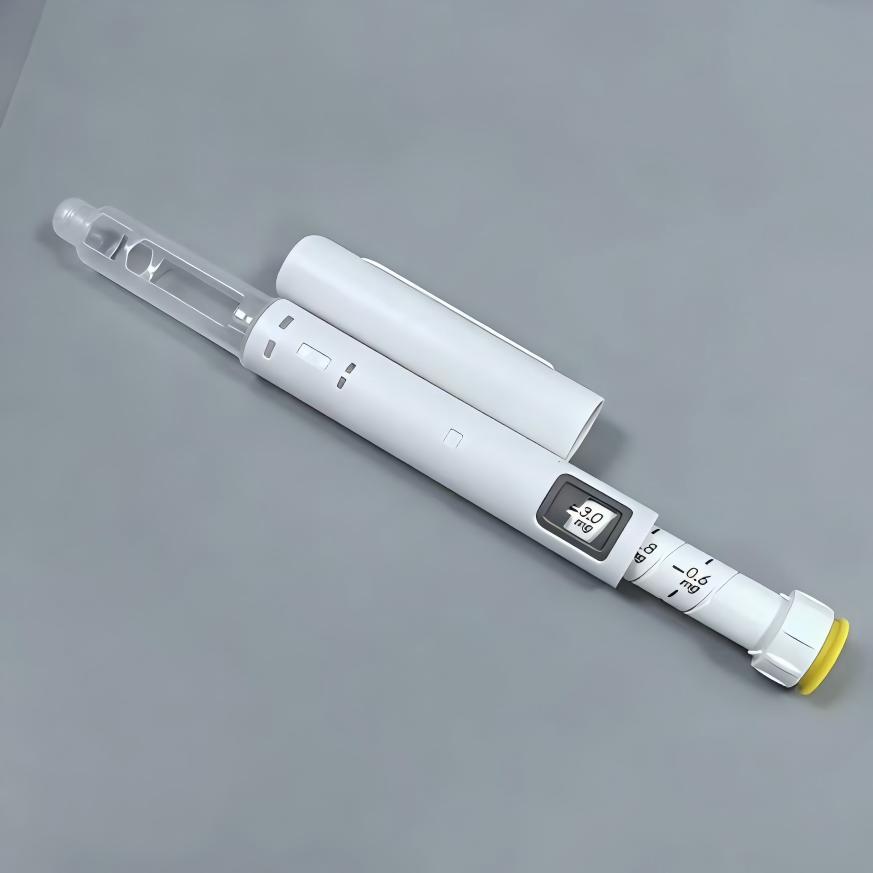
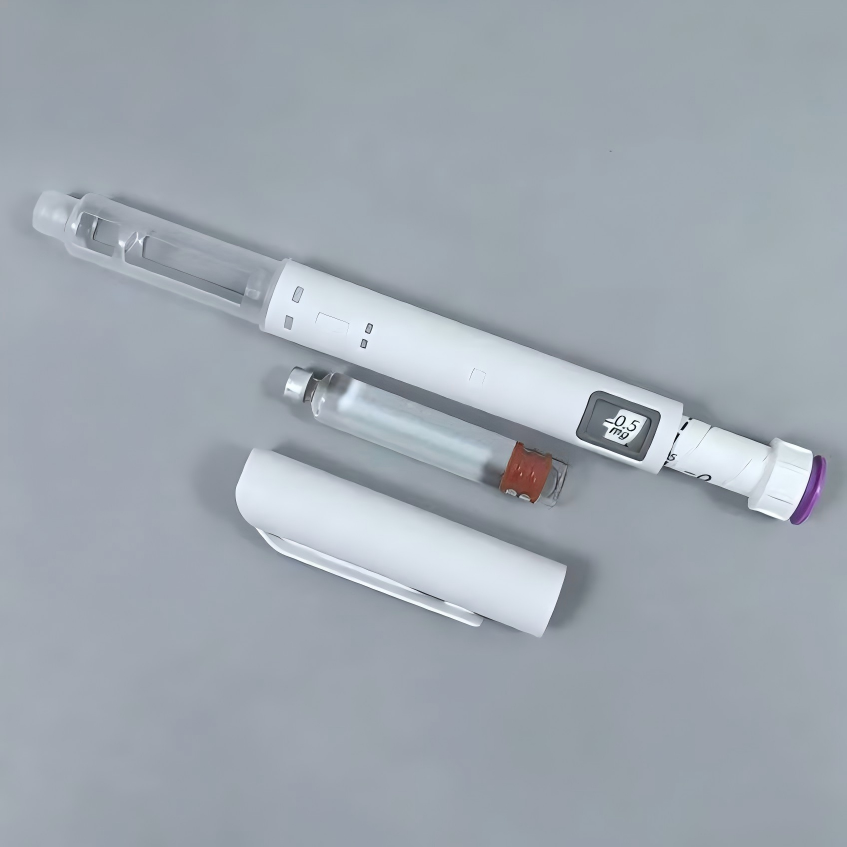
Description
| Pen Injector Type | Dosage Unit | PN | Cartridge (Minimum Dosage Unit) | Laser-etched Scale (Common Dosage) | Main Applicable Drugs |
| XXXXX Series of Disposable Pen Injectors | 37 U | BH0037-SP | 3.0 mL(0.01 mL) | 0-0.25 mg | Semaglutide 1.34 mg/mL concentration (0.5 mg dose) |
| 0-0.5 mg | |||||
| 0-0.25-0.5 mg | |||||
| 50 U | BH0050-SP | 3.0 mL(0.01 mL) | 0-0.6-1.2-1.8-2.4-3.0mg | Liraglutide Injection | |
| 60 U | BH0060-SP | 3.0 mL(0.01 mL) | 0-60(Full) | Insulin Aspart, Insulin Glargine | |
| 72 U | BH0072-SP | 3.0 mL(0.01 mL) | 0-0.25-0.5-1.0 mg | Semaglutide (various concentrations: 0.68 mg/mL, 1.34 mg/mL, 2.68 mg/mL, etc.) | |
| 74 U | BH0074-SP | 3.0mL(0.01 mL) | 0-0.25-0.5 mg | Insulin Aspart, Insulin Glargine. Semaglutide (various concentrations: 0.68 mg/mL, 1.34 mg/mL, 2.68 mg/mL, etc.) | |
| 0-0.25-0.5-1.0 mg | |||||
| 0-2.0 mg | |||||
| 0-1.0 mg | |||||
| 75 U | BH0075-SP | 3.0 mL(0.01 mL) | 0-1.0 mg | Semaglutide | |
| 0-1.7 mg | |||||
| 0-2.4 mg | |||||
| 0-2.0 mg | |||||
| 0-1.7-2.4 mg | |||||
| 0-2.5-5-10 mg | |||||
| 0-1 | |||||
| 80 U | BH0080-SP | 3.0 mL(0.01 mL) | 0-80(Full) | Insulin Degludec/Aspart Series | |
| 3.0 mL(0.0067 mL) | / | ||||
| 1.5 mL(0.0033 mL) | / |
| Basic Info. | ||||
| Model NO. | HL100S-D | Gender | Unisex | |
| Side Effect | No | Usage | For External Use | |
| Pharmaceutical Technology | Chemical Synthesis | Efficacy | Weight Loss & Slimming | |
| Material | Plastic | Max Dosage | 0.6ml | |
| Min Dosage | 0.01ml | Volume | 3ml Cartridges | |
| Use of Mothod | Subcutaneous | Certification | CE/FDA 510K/Fto/Cde | |
| Applicative Drug | Sema/Tirz/Reta/Insulin | Pen Size | 173mm*φ 19.57mm | |
| Transport Package | Bag/Box | Specification | 60u | |
| Trademark | Customization | Origin | China | |
| HS Code | 9018310000 | Production Capacity | 1billion/Year Currently | |
| Device Features | |||
| Highly customizable: appearance, color, dosage, scale lines customizable. | |||
| Currently available in 36/ 37 /50 /60 /74 /75 /80 units, which can be customized for different indications. | |||
| Pressing force: 36/37 units <5N, 74/80 units <8N. | |||
| Accurate dosage, professional R&D team and data validation support. | |||
| Completed the FTO review in China and the United States, no patent risk. | |||
| Applicable to liraglutide, simethicone, follicle stimulating hormone | |||
| (FSH) drugs, insulin degludec and other drugs. | |||
| Successfully applied for CDE number; CE MDR, FDA (510k) has been submitted for review. |