Description
| Product Name |
Max.Single Dose |
Model NO. |
For Cartridge |
Min.Single Dose |
Usual Dose |
Usual Scale Units |
Applicative Drug |
| Reusable Pen Injector |
60U |
BH0060-R01 |
3mL |
0.01mL |
0-1-2-…-60 even number |
IU |
Insulin Aspart series |
| 60U |
BH0060-R02 |
3mL |
0.0075mL |
0-2-…-60 even number |
IU |
Human Growth Hormone |
| 75U |
BH0075-R |
3mL |
0.01mL |
0-1mg;0-1.7mg;0-2.4mg |
mg |
GLP-1 medicine, Peptides |
| 80U |
BH0080-R |
3mL |
0.01mL |
0-2-…-80 |
IU |
Insulin degludec/insulin aspart,IDegAsp;Insulin glargine;Insulin glulisine |
| Basic Info. |
| Model NO. |
BH0060-R01 |
Gender |
Male & Famale |
| Side Effect |
No |
Usage |
For External Use |
| Pharmaceutical Technology |
Chemical Synthesis |
Efficacy |
Weight Loss & Slimming |
| Reusable/Disposable |
Reusable |
Cartridge |
3ml |
| Max Dose |
0.6ml |
Transport Package |
Carton Box |
| Specification |
16.3cm |
Origin |
China |
| Production Capacity |
35million/Year Currently |
|
|
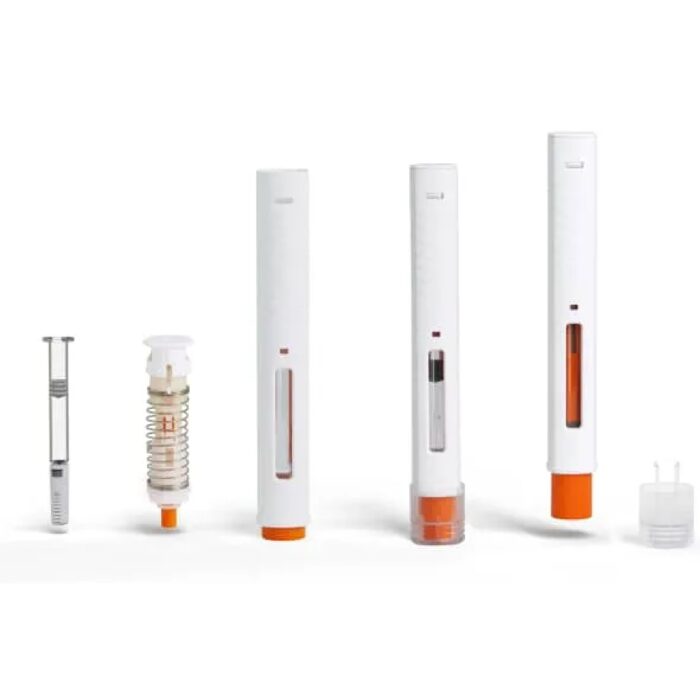
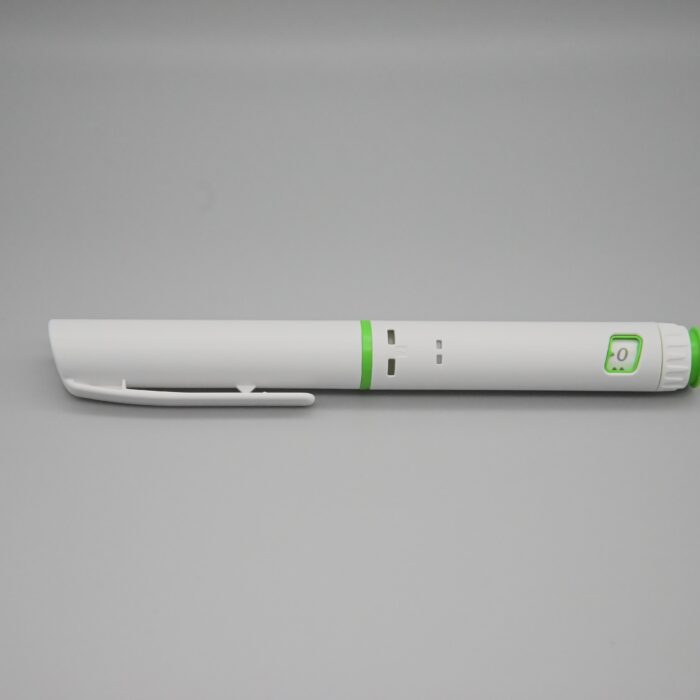
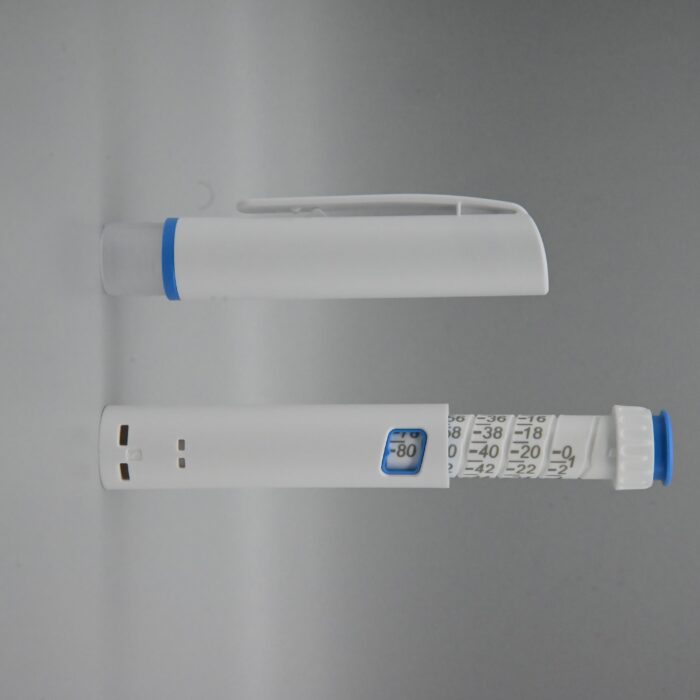
Pen Injector is a type of reusable liquid medicine injection device that contain liquid medicine solution with a syringe. The Liquid medicine medication is placed in the pen in a cartridge, eliminating the need to extract liquid medicine each time.To administer the medication, simply remove the pen cap, adjust the dial to the desired dose, and then perform the liquid medicine injection.
In addition to the common insulin injection, BioauraHealth Pen Injector can also be used for growth hormone, antibiotics, interferon, beauty drugs, anti-biochemical first aid, hemostasis and pain, heart disease first aid, detoxification, antipyretic analgesia, anesthesia and sedation.
| Simple operation |
| It is easy for beginners to use, eliminating the need for professional guidance. |
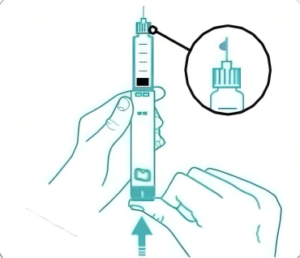

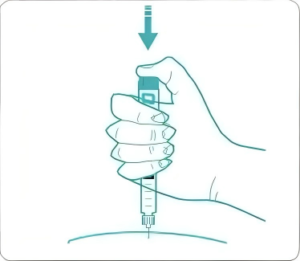
| Device Features |
| 1、Highly customizable: appearance, color, dosage, scale customizable. |
| 2、Current smallest is 0.75 units (0.0075mL) and the injection range covers 60 / 74/80 units; can develop according to different indications. |
| 3、Pressing force: 80 units <15N, 60 units <8N, accurate dosage, professional R&D team and data validation information support. |
| 4、Successfully obtained Class II medical device license; CE MDR, FDA (510k) has been submitted for review. |
| 5、Used for long-term injection of insulin and other drugs. |
| Areas of care: |
| Diabetes-insulin / GLP-1 peptide |
| Osteoporosis |
| Growth Hormone Deficiency |
| Parkinson disease |
| Oncology |
| Rheumatoid arthritis |
| Endocrinology |
| Fertility… |